Article Directory
Of all the revolutions that have shaped human history—the agricultural, the industrial, the digital—the most profound might be the one happening right now, silently, inside petri dishes and supercomputers. It’s a revolution powered by the oldest and most elegant machines in the universe: enzymes.
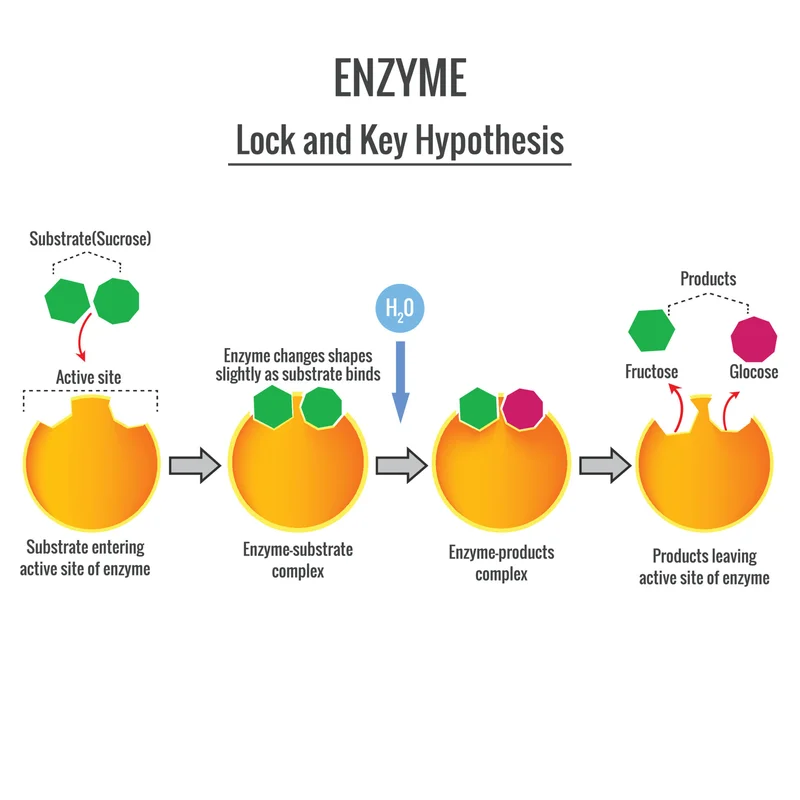
What is an enzyme? At its simplest, it’s a type of protein that acts as a biological catalyst. Think of it as a microscopic specialist, a tiny, purpose-built robot designed by billions of years of evolution to do one specific job with breathtaking efficiency. Enzymes are the tireless workers that replicate your DNA, break down the glucose from your lunch to create energy (ATP), and power every single process that makes you, well, you. For centuries, we’ve only been able to watch these masters at work. But that’s all changing. We’re moving from being spectators of biology to becoming its architects. We’re on the verge of learning to design, build, and deploy our own custom enzymes to solve humanity's biggest problems.
This isn’t just an incremental step forward. It’s a paradigm shift.
The Architects of Biology
For the longest time, finding the right enzyme for a job was like searching for a single, magical key in a landfill the size of a continent. You hoped you’d stumble upon a useful one in some obscure microbe. Now, we’re becoming locksmiths. We’re learning to design the key from scratch.
This leap is being driven by a convergence of three incredible fields. First, we have computational biology, which gives us tools like B-factor analysis and Ancestral Sequence Reconstruction, or ASR. This uses a kind of biological archaeology—in simpler terms, it means we can resurrect the ancient, hyper-stable versions of enzymes that existed in long-extinct organisms and use their robust blueprints to build better ones today. Combined with machine learning and AI-powered tools like AlphaFold2 that can predict an enzyme structure from its genetic code, we can finally move beyond trial and error. We can identify the precise points in a protein to tweak for more stability or a different function.
But a great design is useless if you can’t test it quickly. That’s where the second breakthrough comes in: high-throughput screening. I was recently reading about a new method out of ETH Zurich for Developing drugs—with tens of thousands of minuscule droplets on a small glass plate that just blew my mind. Imagine a small glass plate, submerged in a shallow bath of oil. On it, a robotic arm deposits tens of thousands of minuscule droplets in minutes, each one a self-contained miniature laboratory. You can see them shimmering under the light, a tiny galaxy of experiments, each testing a different enzyme variant or a potential drug. This allows researchers to gather more data in a single afternoon than they once could in a year.
The combination of intelligent design and lightning-fast testing creates a feedback loop that is accelerating discovery at a dizzying pace. What does this new power actually let us do? The answer is where science fiction starts to become reality.
Rewriting the Rules of Medicine
When I first read about how UBC enzyme technology clears first human test toward universal donor organs for transplantation, I honestly just sat back in my chair, speechless. This is the kind of breakthrough that reminds me why I got into this field in the first place. For years, organ transplantation has been shackled by the tyranny of blood types. A patient with type O blood can only receive a type O organ, leading to agonizingly long waits while perfectly good organs from other blood types go unused for them.
Researchers at the University of British Columbia developed a pair of enzymes that act like molecular scissors. The sugars, or antigens, that define blood types coat the blood vessels of our organs like little nametags. These enzymes are designed to find the "Type A" nametag—the substrate, in technical terms—and snip it right off at the active site, revealing the universal "Type O" underneath. In a landmark experiment, they took a human kidney, perfused it with these enzymes, and transplanted it into a brain-dead recipient. For two days, it worked perfectly, with no signs of the violent rejection that would normally destroy an incompatible organ in minutes.
Think about what this means—no more agonizing waits on transplant lists because of blood type, the potential to save thousands of lives is just sitting right there in this clever application of a biological catalyst and it's a testament to what happens when we finally start speaking nature's language. What other biological "rules" that we've always accepted as fixed are suddenly open to negotiation?
This control works both ways. We can use enzymes as tools, but we can also learn to switch them off when they’re causing harm. In Australia, scientists recently identified two enzymes that act as bodyguards for prostate cancer cells, protecting them from treatment and helping them thrive. By developing drugs that specifically block these two culprits, they were able to kill cancer cells and even shrink tumors in animal models. Instead of the chemical sledgehammer of chemotherapy, this is molecular surgery—precise, targeted, and far less damaging to the patient.
Of course, with this incredible power to edit and direct the very machinery of life comes a profound responsibility. We are no longer just discovering nature's secrets; we are actively rewriting them. Every step forward requires us to ask not only "Can we do this?" but "Should we?" The ethical guardrails we build now will determine the world our children inherit. This moment feels like the dawn of the semiconductor. Just as the simple transistor unlocked the entire digital age, these engineered enzymes are poised to unlock a biological one, where our ability to heal, build, and sustain is limited only by our imagination.
Biology is Now Programmable
For all of human history, we’ve built our world with inert materials—with stone, steel, and silicon. We’ve been limited by the laws of physics and chemistry. Now, we’re tapping into a new operating system: life itself. The work being done with enzymes isn't just about making better medicines or greener industrial processes. It’s the first chapter in a new story where we learn to program biology with the same precision with which we program computers. We are on the cusp of a future that won't just be built; it will be grown.
